Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment. How does the product of this reaction promote the formation of the
By A Mystery Man Writer
Last updated 02 Jun 2024

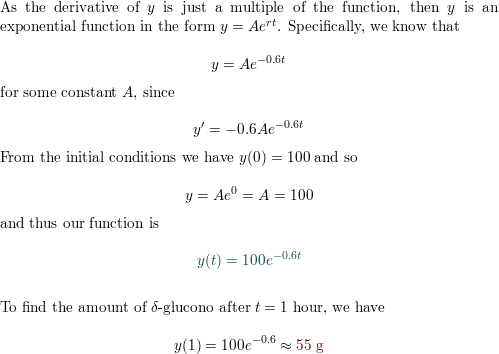
First-order chemical reactions In some chemical reactions, t

Synthesis and Interface Activity of a Series of Carboxylic Quaternary Ammonium Surfactants in Hydraulic Fracturing
Interactions of the Kaolin Minerals with Complex Organic Molecules
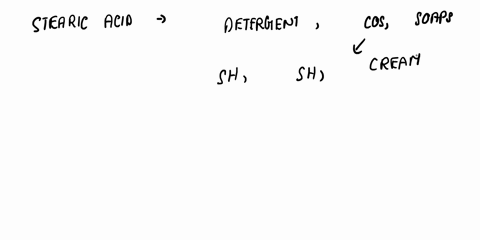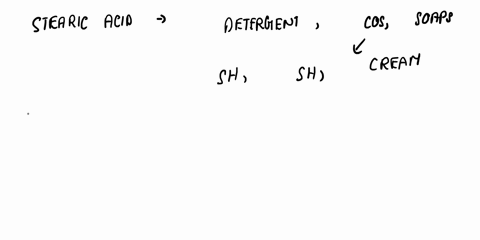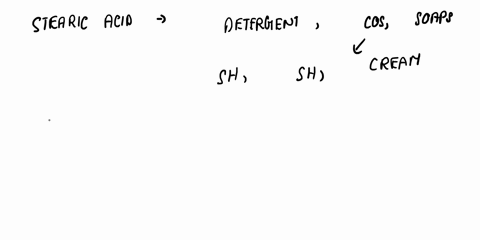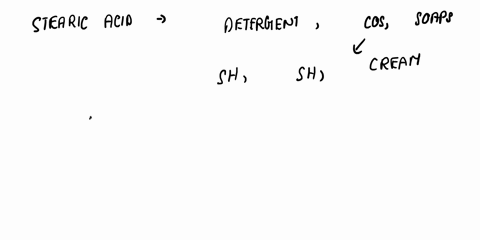
SOLVED: a) Is stearic acid a necessary ingredient for the
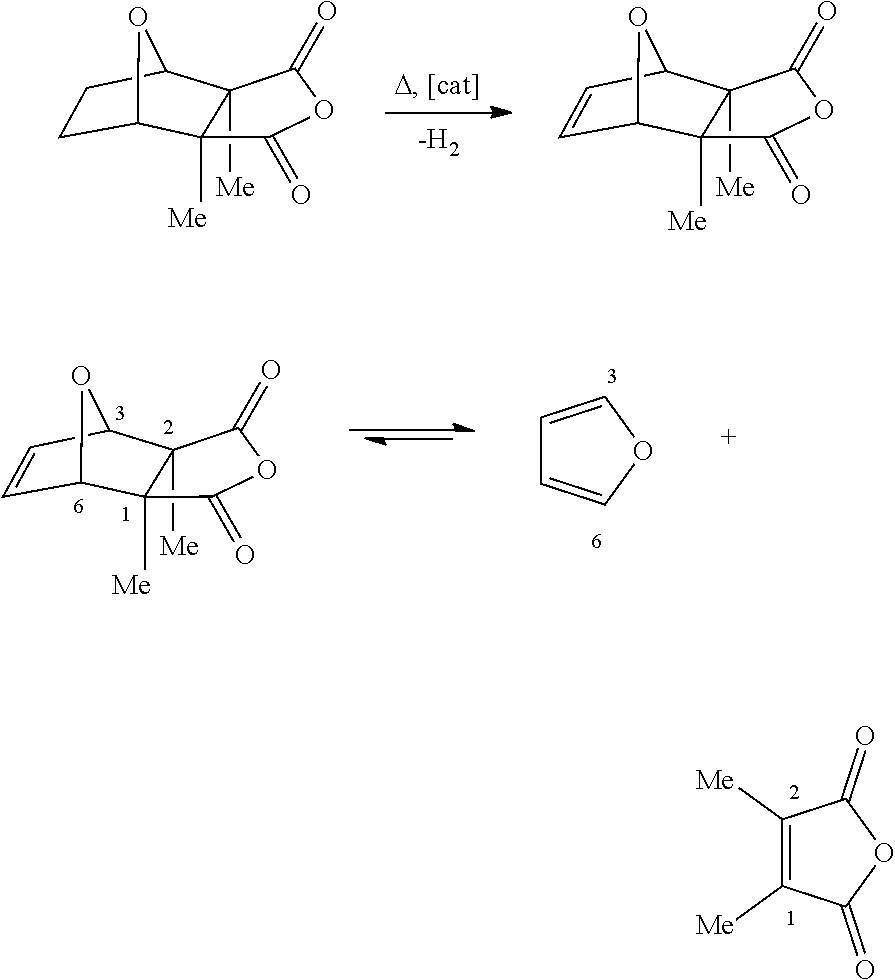
Synthesis Of Cantharidin Davidson; Matthew Gene ; et al. [Verrica Pharmaceuticals, Inc.]
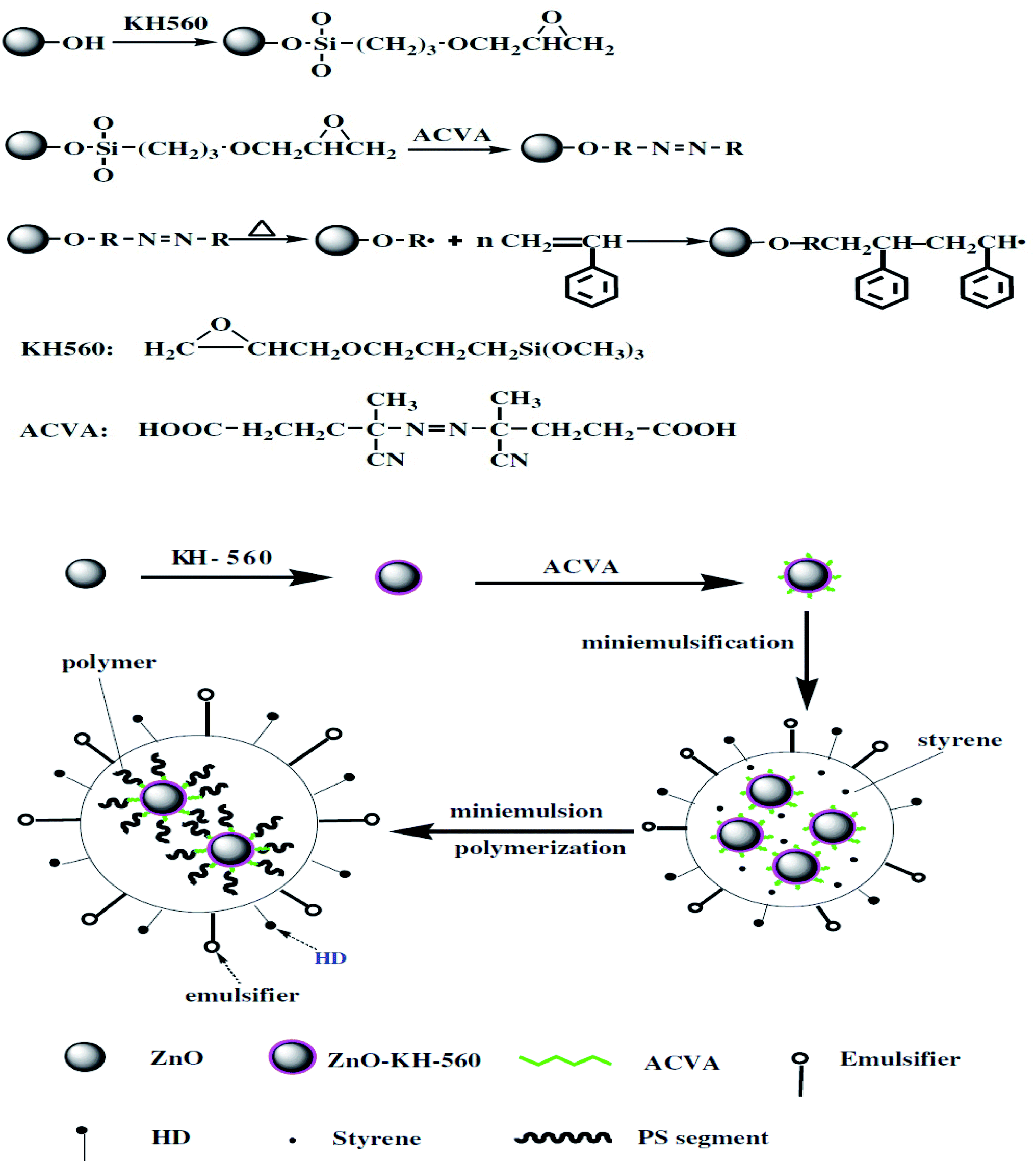
ZnO nanostructured materials and their potential applications: progress, challenges and perspectives - Nanoscale Advances (RSC Publishing) DOI:10.1039/D1NA00880C
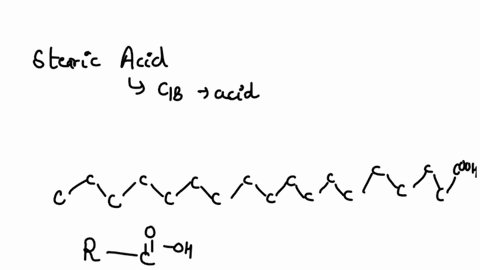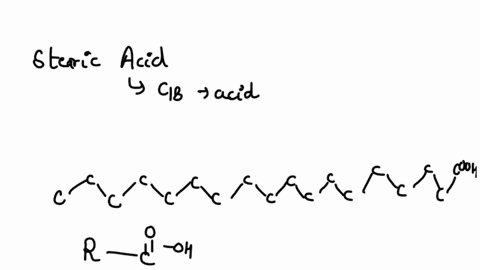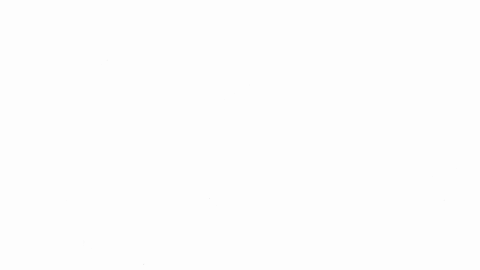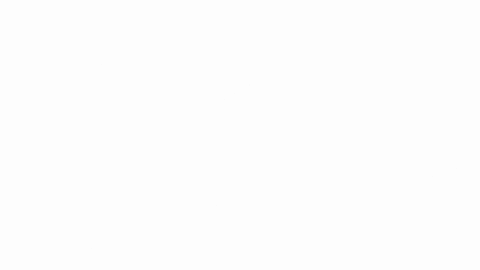
SOLVED: Arrow pushing Mechanism: Please based on your observations

Interaction of the Acid Soap of Triethanolamine Stearate and Stearic Acid with Water

PDF) Characterising the phase behaviour of stearic acid and its triethanolamine soap and acid–soap by infrared spectroscopy

SOLVED: c) Write a chemical equation for the reaction that occurs

Interaction of the Acid Soap of Triethanolamine Stearate and Stearic Acid with Water
Recommended for you
-
 Beginner's Guide to Emulsifiers - Oh, The Things We'll Make!02 Jun 2024
Beginner's Guide to Emulsifiers - Oh, The Things We'll Make!02 Jun 2024 -
 A Quick Guide to Stearic Acid & Liquid Oil Ratios - Humblebee & Me02 Jun 2024
A Quick Guide to Stearic Acid & Liquid Oil Ratios - Humblebee & Me02 Jun 2024 -
 Stearic Acid - Summer Rain02 Jun 2024
Stearic Acid - Summer Rain02 Jun 2024 -
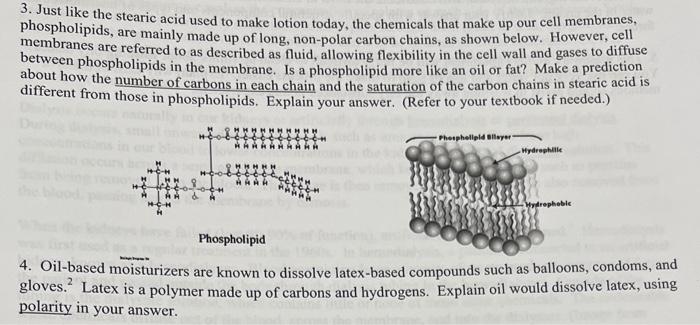
Solved 3. Just like the stearic acid used to make lotion02 Jun 2024
-
 Charco's Stearic Acid White Flakes NF/USP - (Triple Pressed) Natural preservative, thickener, stabilizer for soap, lotion, cream {(C18H36O2), CAS No. 57-11-4} (250 Gm) : Buy Online at Best Price in KSA02 Jun 2024
Charco's Stearic Acid White Flakes NF/USP - (Triple Pressed) Natural preservative, thickener, stabilizer for soap, lotion, cream {(C18H36O2), CAS No. 57-11-4} (250 Gm) : Buy Online at Best Price in KSA02 Jun 2024 -
 Talk It Out Tuesday: All Things Lotion - Soap Queen02 Jun 2024
Talk It Out Tuesday: All Things Lotion - Soap Queen02 Jun 2024 -
 Lavender & Chamomile Body Lotion – Homestead Princeton02 Jun 2024
Lavender & Chamomile Body Lotion – Homestead Princeton02 Jun 2024 -
 Tallow Lotion Recipe: Light, Non Greasy - Bumblebee Apothecary02 Jun 2024
Tallow Lotion Recipe: Light, Non Greasy - Bumblebee Apothecary02 Jun 2024 -
 Nourishing Lotion with Cocoa Shea02 Jun 2024
Nourishing Lotion with Cocoa Shea02 Jun 2024 -
 Learn How To Make Cosmetics - Beginner Lotion Recipe - Summer Rain02 Jun 2024
Learn How To Make Cosmetics - Beginner Lotion Recipe - Summer Rain02 Jun 2024
You may also like
-
 Black Leather Choker Necklace – Viviane Guenoun02 Jun 2024
Black Leather Choker Necklace – Viviane Guenoun02 Jun 2024 -
 40 Pcs Bubble Bell Floral Foam Ball Christmas Tree Ornaments Decorations Locket02 Jun 2024
40 Pcs Bubble Bell Floral Foam Ball Christmas Tree Ornaments Decorations Locket02 Jun 2024 -
/product/39/854176/2.jpg?8852) Soulbyee Teeth Repair Kit, Moldable False Teeth, Temporary Teeth02 Jun 2024
Soulbyee Teeth Repair Kit, Moldable False Teeth, Temporary Teeth02 Jun 2024 -
new jellies!! #fyp #bead #jewellery #beadhaul #jellyfish, jellyfish02 Jun 2024
-
 Vintage Journaling Acrylic Stamp A – Papergame02 Jun 2024
Vintage Journaling Acrylic Stamp A – Papergame02 Jun 2024 -
 Kraft Shipping Tubes 2 1/2 X 24 - 0.070 Thick | Quantity: 2502 Jun 2024
Kraft Shipping Tubes 2 1/2 X 24 - 0.070 Thick | Quantity: 2502 Jun 2024 -
 Winsor & Newton Liquin Light Gel Medium 8.5 oz.02 Jun 2024
Winsor & Newton Liquin Light Gel Medium 8.5 oz.02 Jun 2024 -
 6 Yards Braid Gimp Trim Braided Cord Scalloped Edge Braid Rick Rack Trim For Sewing, Pillows, Home Curtain - Temu Japan02 Jun 2024
6 Yards Braid Gimp Trim Braided Cord Scalloped Edge Braid Rick Rack Trim For Sewing, Pillows, Home Curtain - Temu Japan02 Jun 2024 -
 Harloon 30 Sets Employee Appreciation Gifts Thank You Gift02 Jun 2024
Harloon 30 Sets Employee Appreciation Gifts Thank You Gift02 Jun 2024 -
 2 Crayola Construction Paper Royalty-Free Images, Stock Photos & Pictures02 Jun 2024
2 Crayola Construction Paper Royalty-Free Images, Stock Photos & Pictures02 Jun 2024